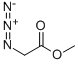
PRODUCT Properties
| Boiling point: | 35 °C |
| Density | 1.182 g/mL at 25 °C |
| refractive index | n20/D1.440 |
| Flash point: | 48℃ |
| form | liquid |
| InChI | InChI=1S/C3H5N3O2/c1-8-3(7)2-5-6-4/h2H2,1H3 |
| InChIKey | RXYCUKSOYJWGPE-UHFFFAOYSA-N |
| SMILES | C(=O)(OC)CN=[N+]=[N-] |
Description and Uses
- Methyl 2-azidoacetate is widely used as a precursor to build triazole derivatives via alkyne-azide click chemistry. Some of the examples include the synthesis of coumarin-triazole derivatives as potential antiplasmodial agents and macrocyclic triazole containing largazole analogs as histone deacetylases-1 (HDAC1) inhibitors.
- It can be used to synthesize various pyrrole derivatives by Knoevenagel condensation as in the synthesis of near-infrared boron dipyrromethene (BODIPY) donors for organic tandem solar cells.
- It can also be used in Hemetsberger-Knittel synthesis of substituted 5-, 6-, and 7-azaindoles.
- Synthesis of highly fluorescent pyreno[2,1-b]pyrroles using methyl 2-azidoacetate as one of the reagents.
Safety
| Symbol(GHS) |   GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226-H315-H319-H335 |
| Precautionary statements | P261-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 5-10-36/37/38 |
| Safety Statements | 26 |
| RIDADR | UN 1993C 3 / PGIII |
| WGK Germany | 3 |
| HazardClass | 3 |
| Limited Quantities | 5.0 L (1.3 gallons) (liquid) |
| Excepted Quantities | Max Inner Pack (30g or 30ml) and Max Outer Pack (1Kg or 1L) |