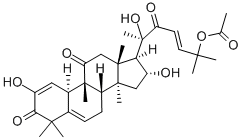
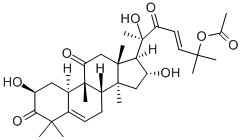
CucurbitacinE , >99% , 18444-66-1
Synonym(s):
α-Elaterin;α-Elaterine
CAS NO.:18444-66-1
Empirical Formula: C32H44O8
Molecular Weight: 556.69
MDL number: MFCD00135936
EINECS: 242-325-2
| Pack Size | Price | Stock | Quantity |
| 1mg | RMB317.60 | In Stock |
|
| 5mg | RMB799.20 | In Stock |
|
| 10mg | RMB1359.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 228-234°C |
| alpha | D -59° (c = 0.7 in chloroform) |
| Boiling point: | 545.56°C (rough estimate) |
| Density | 1.1059 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble15mg/mL, clear |
| form | powder |
| pka | 8.51±0.70(Predicted) |
| color | white to beige |
| optical activity | [α]/D -60 to -75°, c = 0.7 (CDCl3) |
| InChIKey | NDYMQXYDSVBNLL-MUYMLXPFSA-N |
| SMILES | [C@@]12(C)C[C@@H](O)[C@]([H])([C@](O)(C)C(=O)/C=C/C(C)(C)OC(=O)C)[C@@]1(C)CC(=O)[C@@]1(C)[C@]3([H])C=C(C(=O)C(C)(C)C3=CC[C@@]21[H])O |&1:0,3,5,7,21,26,28,40,r| |
| LogP | 3.150 (est) |
Description and Uses
Cucurbitacin E is a plant-derived triterpene that has diverse biological activities. At a concentration of 10 pM, it reduces MPP+-induced death of neuronal PC12 cells through inhibition of autophagy in vitro. Cucurbitacin E inhibits growth of T24 bladder, MDA-MB-468 and MCF-7 breast, PC3 prostate, and colorectal cancer cell lines (IC50s = 50-1,000 nM) through induction of G2/M arrest and apoptosis. It increases bilirubin binding to human serum albumin (HSA) in human plasma in a dose-dependent manner. Cucurbitacin E also inhibits depolymerization of actin filaments isolated from rabbit skeletal muscle actin and in HeLa cells.
Cucurbitacin E has been used as a cofilin inhibitor. It is also used as a F-actin stabilizer to prevent membrane-associated periodic skeleton (MPS) loss and protect from axonal fragmentation.
Safety
| Symbol(GHS) |  GHS07 |
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P301+P312+P330 |
| Hazard Codes | Xn |
| Risk Statements | 25-22 |
| Safety Statements | 1-22-45-24/25 |
| RIDADR | 3172 |
| WGK Germany | 3 |
| RTECS | RC6305500 |
| HS Code | 29153900 |
| Storage Class | 11 - Combustible Solids |
| Hazard Classifications | Acute Tox. 4 Oral |
| Toxicity | LD50 orally in mice: 340 mg/kg (Albert) |