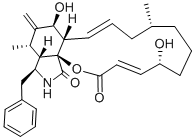
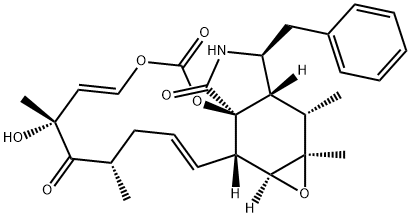
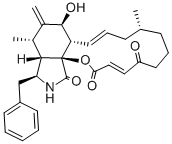
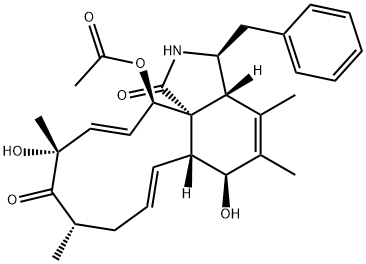
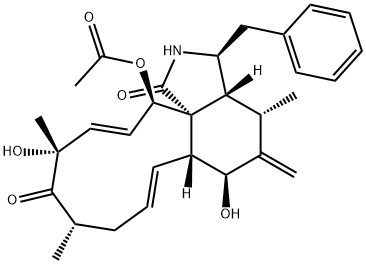
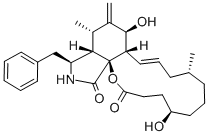
Cytochalasin B , 98% , 14930-96-2
Synonym(s):
Cytochalasin B, Helminthosporium dematioideum - CAS 14930-96-2 - Calbiochem;Phomin
CAS NO.:14930-96-2
Empirical Formula: C29H37NO5
Molecular Weight: 479.61
MDL number: MFCD00077704
EINECS: 239-000-2
| Pack Size | Price | Stock | Quantity |
| 1MG | RMB557.60 | In Stock |
|
| 5MG | RMB1519.20 | In Stock |
|
| 25mg | RMB5439.20 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 218-223 °C |
| Boiling point: | 577.96°C (rough estimate) |
| Density | 1.1490 (rough estimate) |
| refractive index | 1.6290 (estimate) |
| Flash point: | 85℃ |
| storage temp. | -20°C |
| solubility | ethanol: 20 mg/mL |
| form | powder |
| pka | 13.60±0.70(Predicted) |
| color | white |
| Merck | 13,2819 |
| BRN | 1096207 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| InChIKey | UXVDBQLLXVKPRS-UZBFCAAUSA-N |
| SMILES | C[C@@H]1CCC[C@@H](O)\C=C\C(=O)O[C@]23[C@@H](\C=C\C1)[C@H](O)C(=C)[C@@H](C)[C@H]2[C@H](Cc4ccccc4)NC3=O |
| LogP | 3.370 |
| EPA Substance Registry System | Cytochalasin B (14930-96-2) |
Description and Uses
Cytochalasin B (14930-96-2) is a cell permeable fungal toxin which binds to the barbed end of actin, inhibiting its polymerization.1 Inhibits cell division, migration and glucose transport.2 Causes cell cycle arrest at G2/M and induces apoptosis in HCT-116 colorectal carcinoma cells.3? Cytochalasin B-induced membrane vesicles (CIMVs) retain cell surface receptors of the parent cells and retain fusion specificity with target cells.4 CIMVs are a promising new vector system for drug and biomolecule delivery due to their natural origin and participation in intercellular communication.5
Cytochalasin B is one of the most extensively studied members of a family of potent mycotoxins produced by several species of fungi. All members of the class exhibit profound effects on cytoskeletal proteins, giving rise to pronounced morphogenic activity in animals and plants. Like most cytochalsins, cytochalasin B exhibits potent inhibition of actin filament function leading to cell death by apoptosis, and displays a broad range of resultant cellular actions. Despite the common mode of action, there is evidence that individual members of this class display diverse selectivity. However, lack of comparative co-metabolite analysis has restricted a more complete understanding of their individual selectivity.
Safety
| Symbol(GHS) |   GHS06,GHS08 |
| Signal word | Danger |
| Hazard statements | H300+H310+H330-H361 |
| Precautionary statements | P201-P202-P260-P280-P302+P352+P310-P304+P340+P310 |
| PPE | Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+,T |
| Risk Statements | 26/27/28-63-23/24/25 |
| Safety Statements | 28-36/37-45 |
| RIDADR | UN 1544 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | RO0205000 |
| F | 10 |
| HazardClass | 6.1(a) |
| PackingGroup | I |
| HS Code | 29349990 |
| Storage Class | 6.1A - Combustible acute toxic Cat. 1 and 2 very toxic hazardous materials |
| Hazard Classifications | Acute Tox. 1 Inhalation Acute Tox. 2 Dermal Acute Tox. 2 Oral Repr. 2 |
| Hazardous Substances Data | 14930-96-2(Hazardous Substances Data) |
| Toxicity | LD50 intraperitoneal in mouse: 30mg/kg |