Triflupromazine Hydrochloride , >98.0%(HPLC) , 1098-60-8
Synonym(s):
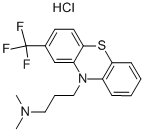
N,N-Dimethyl-2-(trifluoromethyl)-10H-phenothiazine-10-propanamine monohydrochloride
CAS NO.:1098-60-8
Empirical Formula: C18H20ClF3N2S
Molecular Weight: 388.88
MDL number: MFCD00058103
EINECS: 214-149-6
| Pack Size | Price | Stock | Quantity |
| 1g | RMB54.40 | In Stock |
|
| 5G | RMB160.80 | In Stock |
|
| 25G | RMB472.00 | In Stock |
|
| others | Enquire |
PRODUCT Properties
| Melting point: | 174.0 to 179.0 °C |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| Water Solubility | Soluble in water |
| solubility | DMF: 5mg/mL; DMSO: 3mg/mL; Ethanol: 5mg/mL; Ethanol:PBS (pH 7.2) (1:10): 0.09mg/mL |
| color | White to Light yellow |
| λmax | 305nm(H2O)(lit.) |
| Merck | 14,9685 |
| BRN | 3801519 |
| Stability: | Hygroscopic |
| Major Application | pharmaceutical (small molecule) |
| InChI | 1S/C18H19F3N2S.ClH/c1-22(2)10-5-11-23-14-6-3-4-7-16(14)24-17-9-8-13(12-15(17)23)18(19,20)21;/h3-4,6-9,12H,5,10-11H2,1-2H3;1H |
| InChIKey | FTNWXGFYRHWUKG-UHFFFAOYSA-N |
| SMILES | Cl.CN(C)CCCN1c2ccccc2Sc3ccc(cc13)C(F)(F)F |
| CAS DataBase Reference | 1098-60-8 |
Description and Uses
Triflupromazine is a phenothiazine with diverse biological activities. It binds to muscarinic receptors in isolated rat corpus striatum (IC50 = 100 μM in a radioligand binding assay). Triflupromazine inhibits serotonin (5-HT) uptake by isolated rat brainstem synaptosomes (IC50 = 0.8 μM). It inhibits T. cruzi infection in mouse peritoneal macrophages when used at a concentration of 12.5 μM. Triflupromazine is active against S. aureus, shigellae, and vibrios (MICs = 2-100 μg/ml) in vitro and is protective against S. typhimurium infection in mice when administered at a dose of 30 μg per animal. Formulations containing triflupromazine were previously used as antipsychotics.
analgesic, antiinflammatory, antipyretic, COX-II inhibitor
Safety
| Symbol(GHS) |  GHS06 |
| Signal word | Danger |
| Hazard statements | H301-H312+H332 |
| Precautionary statements | P261-P264-P280-P301+P310-P302+P352+P312-P304+P340+P312 |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22 |
| Safety Statements | 36 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | SO8925000 |
| HazardClass | 6.1 |
| PackingGroup | III |
| HS Code | 2934302300 |
| Storage Class | 6.1C - Combustible acute toxic Cat.3 toxic compounds or compounds which causing chronic effects |
| Hazard Classifications | Acute Tox. 3 Oral Acute Tox. 4 Dermal Acute Tox. 4 Inhalation |