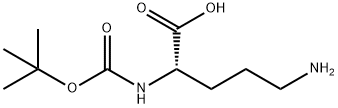
A1228212
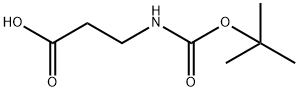
Boc-β-Ala-OH , 98% , 3303-84-2
Synonym(s):
Boc-β-alanine
CAS NO.:3303-84-2
Empirical Formula: C8H15NO4
Molecular Weight: 189.21
MDL number: MFCD00037291
EINECS: 221-979-2
| Pack Size | Price | Stock | Quantity |
| 5G | RMB24.00 | In Stock |
|
| 10G | RMB35.20 | In Stock |
|
| 25G | RMB60.80 | In Stock |
|
| 100G | RMB210.40 | In Stock |
|
| 500g | RMB753.60 | In Stock |
|
| others | Enquire |
Update time: 2022-07-08
PRODUCT Properties
| Melting point: | 76-78 °C |
| Boiling point: | 333.9±25.0 °C(Predicted) |
| Density | 1.129±0.06 g/cm3(Predicted) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| pka | 4.46±0.10(Predicted) |
| form | Crystalline Powder |
| color | White to off-white |
| BRN | 1909986 |
| Major Application | peptide synthesis |
| InChI | InChI=1S/C8H15NO4/c1-8(2,3)13-7(12)9-5-4-6(10)11/h4-5H2,1-3H3,(H,9,12)(H,10,11) |
| InChIKey | WCFJUSRQHZPVKY-UHFFFAOYSA-N |
| SMILES | C(=O)(NCCC(=O)O)OC(C)(C)C |
| CAS DataBase Reference | 3303-84-2(CAS DataBase Reference) |
Description and Uses
Boc-beta-Ala-OH is an alkane chain with terminal carboxlic acid and Boc-protected amino groups. The compound can be used as a PROTAC linker in the synthesis of PROTACs. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The Boc group can be deprotected under mild acidic conditions to form the free amine.
Boc-?β-?Ala-?OH is a reagent used in the synthesis of quinazolines as platelet aggregation inhibitors and ligands of integrin.
Safety
| Symbol(GHS) |   GHS07,GHS05 |
| Signal word | Danger |
| Hazard statements | H319-H314-H335 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P260-P264-P280-P301+P330+P331-P303+P361+P353-P363-P304+P340-P310-P321-P305+P351+P338-P405-P501 |
| PPE | Eyeshields, Gloves, type N95 (US) |
| Hazard Codes | Xn |
| Risk Statements | 20/21/22-36/37/38 |
| Safety Statements | 22-24/25-36-26 |
| WGK Germany | 3 |
| HazardClass | IRRITANT |
| HS Code | 29241990 |
| Storage Class | 11 - Combustible Solids |